When there are enterprises catching up on the Digital Transformation, the next disruption that enterprises to be geared up is on the topic ” Intelligent enterprises”
What is Intelligent Enterprise?
There are many definitions, but simple way for me to understand is that organization which are utilizing emerging technologies like Artificial Intelligence (AI), Machine Learning ( ML) and Internet of Things( IOT) to help their business/customer/Product to focus on the High value tasks.
There are many case studies which are emerging and when i have not witnessed anything in person but i am reading about the Robotics used in the Internal movement of goods without any Human intervention. It is like a Point to Point movement of goods with automated signals. Example: A kanban Push / Pull mechanism can be fully automated when the Bins Trigger Full/ Empty signal.
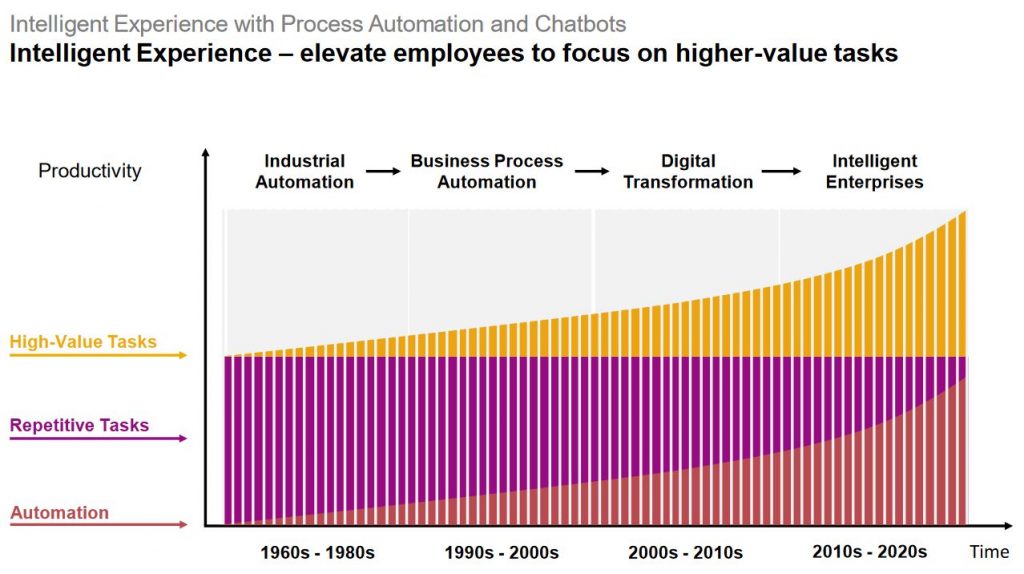
Imagine if an enterprise can provide information to the customer during the complete life cycle from order Placement till delivery of goods and also monitoring the product/service Post delivery. In case if you can also provide flexibility to modify few characteristics during intermittent Stages. I trust then the customer is going to have new experience and the enterprise will also be able to add more value to the product and serivice. If so much flexibility is required then there is lot to be improved in terms of automation, for which adapting the AI,ML,IOT all needs to be colloborated.
I will continue to explore and update this topic. If you have any practical working scenarios will be glad to connect with you.
For more information you can read here https://www.sap.com/india/products/intelligent-enterprise.html